Clinical Trials
Mobile Application
CTMA provides French innovative digital solutions aiming at facilitating patient recruitment in clinical studies, using current patients’ conditions and powerful build-in algorithms.
Our Mission
http://chicken-flavor.fr/wp-content/uploads/2022/07/video-mission-site-web.mp4
Some numbers
The drug development landscape is increasingly complex and competitive, with a high pressure on the time to market.
Figure: Total R&D/clinical trials pipeline size, by year 2000-2022
They are so many drugs in development that it is extremely challenging to finds the way to one of them, as a patient or as a site, or to secure a high visibility on one of them for a Sponsor.
CT-SCOUTTM and ToTem4me facilitate the match between the patients and the studies.
%
Of studies fail to meet patient recruitment deadlines
However, millions of patients visit clinical sites every day.
CT-SCOUT™ help investigators identify the ones that could benefit from a clinical study.
%
OF THE PATIENTS WOULD WELCOME THE OPPORTUNITY TO PARTICIPATE TO A CLINICAL STUDY
Patients have become actors of their treatment conditions.
ToTem4me help them identify potential opportunities through clinical studies.
%
of the patients are being offered to participate to a clinical study
But 80% of patients would accept to participate t a clinical study.
CT-SCOUT™ contributes to increase the percentage of patients considered for clinical research.
Weeks set-up time
No more than 2 weeks are required to get CT-SCOUT™
or ToTem4me deployed for a clinical study
Our Solutions
We have customized digital solutions for the different contributors to the clinical research efforts.
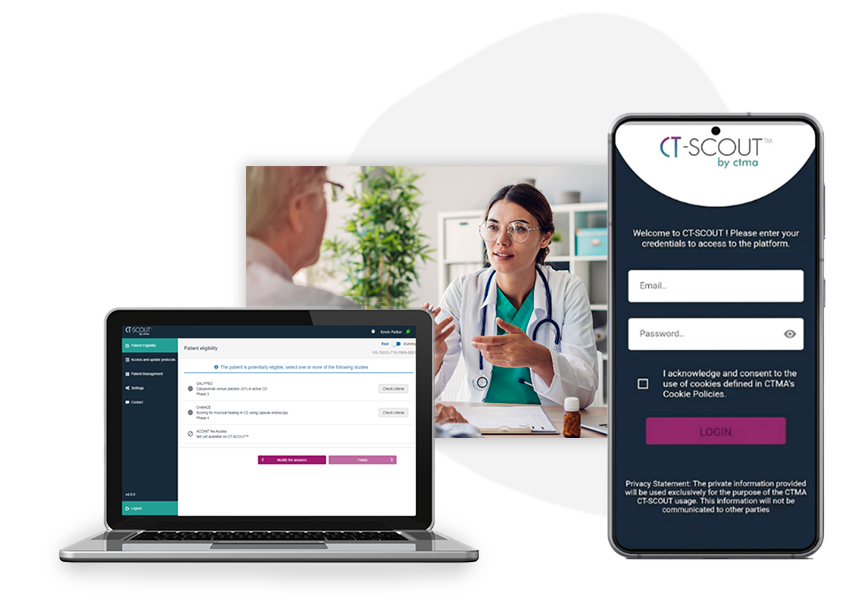

CT-SCOUT
The missing link between sponsor & sites
ToTem4me
The web application to reach your matching studies
DaaS
Coming soon
CT-SCOUT™
The missing link between sponsor & sites
A multi-device application based on a unique algorithmic model to enable all physicians to quickly detect in real-time, the clinical studies running at their sites, for which a patient is potentially eligible according to his/her current health conditions.
ToTem4me
The web application for patients
A multi-device application based on a unique algorithmic model, to enable patients, with the direct support of an expert-coordinator, to quickly detect the clinical studies running in
DaaS Solution
Our teams are currently working on a Data solution, we will keep you informed shortly.
Testimonials
« CT-SCOUT™ represents a true added value to clinical research. It takes only few minutes to operate and study information is updated very quickly »
Prof Stéphane NanceyHepato-gastroenterology department,
Lyon-Sud University Hospital
« CT-SCOUT™ is important for patients because it facilitates their enrolment in clinical trials. Before CT-SCOUT™ we missed out on a lot of patients and now we have dramatically increased accrual in our studies. We communicate on it and this has clearly increased our visibility at national and international levels »
ECCO Congress
Prof Laurent Peyrin-Biroulet,

Prof Laurent Peyrin-BirouletPresident, GETAID and ECCO
Précédent
Suivant
News
You can follow CTMA on Twitter, LinkedIn, and Instagram, but here below you can find an extract of the recent company news !
CTMA IS MEMBERS OF THE FRANCE BIOTECH!
•
juin 10, 2022
CTMA is proud to announce that we have become members of the France BioTech. Click here on the link below to read …
CTMA AU SALON MEDINTECHS!
•
juin 10, 2022
Nous avons le Plaisir de vous annoncer que CTMA sera présente au salon MedInTech le 9 mars prochain, qui se tiendra au …
CTMA AT THE “ 10E JOURNÉE DE RECHERCHE CLINIQUE”
•
juillet 2, 2021
CTMA will be participating at the “ 10e Journée de Recherche Clinique” tomorrow Thursday 15th.Our CEO, Pierre Pellier will pitch for CTMA during an “Elevator …
- + 33 1 48 03 47 84
- 13, Place Kossuth 75009 Paris
Social













